The human gastrointestinal (GI) tract represents one of the largest interfaces (250–400 m2) between the host, environmental factors and antigens in the human body. The collection of bacteria, archaea and eukarya colonising the GI tract is termed the ‘gut microbiota’ and has co-evolved with the host over thousands of years to form an intricate and mutually beneficial relationship.
The development of the microbiota is generally believed to begin from birth, although this dogma is challenged by a limited number of studies in which microbes were detected in womb tissues, such as the placenta. After birth, the GI tract is rapidly colonised, with life events such as illness, antibiotic treatment and changes in diet causing chaotic shifts in the microbiota.
In early stages of development, the microbiota is generally low in diversity and is dominated by two main phyla, Actinobacteria and Proteobacteria. During the first year of life, the microbial diversity increases, and the microbiota composition converges towards a distinct adult-like microbial profile with temporal patterns that are unique to each infant. By around 2.5 years of age, the composition, diversity and functional capabilities of the infant microbiota resemble those of adult microbiota. In more recent years, we’ve come to understand the importance of gut microbes and their role in regulating the immune system. The early microbiome is what trains your immune system. This is extremely important because it is what helps prevent disease and fight infection. Babies have a very clean system, so helping build up their gut health will allow their microbiome to more effectively manage irritants, toxins and other threats. Good gut health not only controls illness but also influences the development of other organs, such as the brain, skin, liver, and kidneys. All of this is vital to the lifetime of better health.
Probiotics are the beneficial bacteria that balance the pathogenic bacteria in our bodies. They are found in different foods, supplements etc. A daily dose of probiotics helps improve gut health and each strain serve a specific role for creating balanced system. Some of the ways in which probiotics help infants are:
- Boosted Immunity – Over 80% of our immune system is in our gut, making this the center of our health & wellness. Balancing your babies gut flora will improve their ability to handle illness, deal with inflammation and improve digestion.
- Ease colic or Reflux – The imbalance of microbiota has adverse effects on babies. Colic, reflux, irregularity of the bowls or constipation can all come from poor gut health.
- Reducing risk of obesity and Asthma – Low levels of healthy bacteria in the GI tract can put people at a greater risk for obesity and asthma. Using a daily probiotic supplement can help reduce the risk of these issues later in life.
Factors Shaping the GI Microbiota
Feeding methods can also affect the abundance of some bacterial groups in the gut microbiota of infants. For example,fucosylated oligosaccharides present in human milk can be utilised by Bifidobacterium longum and several species of Bacteroides allowing them to outcompete other bacteria such as E. coli and Clostridium perfringens. Whilst the abundance of Bifidobacterium spp. in breast-fed infant microbiota is typically high this is reduced in formula-fed infants. Furthermore, formula-fed infant microbiota has an increased diversity and altered levels of other groups such as E. coli, Clostridium difficile, Bacteroides fragilis and lactobacilli. Several environmental factors have been implicated in shaping the microbiota , such as antibiotics but not host-targeted drugs, shape the physiology and gene expression of the active human gut microbiome . Antibiotic treatment dramatically disrupts both short- and long-term microbial balance, including decreases in the richness and diversity of the community. The exact effects and the time for recovery of the microbiota following antibiotic administration appear to be individual-dependent.
Treatment of acute Diarrhea and necrotizing Colic in Infants
Acute diarrhea is a burdensome disease with potentially harmful consequences, especially in childhood. Diarrhea refers to the abrupt onset of three or more loose or liquid stools per day. More specifically, acute diarrhea is defined as an abnormally frequent discharge of semi-solid or fluid fecal matter from the bowel, lasting less than 14 days. Although it is a preventable disease, acute Diarrhea remains a major cause of morbidity and mortality in children worldwide, resulting in 525,000 deaths per year among those younger than five years. Most of these mortalities occur in developing countries. Currently, the World Health Organization (WHO) recommends treatment of acute childhood diarrhea with oral rehydration salts (ORS) and continued feeding for the prevention and treatment of dehydration, as well as zinc supplementation to shorten the duration and severity of the diarrheal episode. Probiotics are living micro-organisms that, upon ingestion in certain numbers, exert health benefits beyond inherent general nutrition. It has been suggested that probiotics modulate the immune response, produce antimicrobial agents, and compete in nutrient uptake and adhesion sites with pathogens. The European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) and the European Society of Pediatric Infectious Diseases (ESPID) currently recommend the use of Saccharomyces boulardii in the management of children with acute Diarrhea as an adjunct to rehydration therapy. Also, a systematic review and meta-analysis of randomized controlled trials conducted by Sanofi Aventis GMbh found Bacillus clausii to be an effective therapeutic option in acute child diarrhea. (Nutrients 2018, 10, 1074).
Future Perspectives
In conclusion, newborn and children possess less diverse and an unstable gut microbial composition which is more susceptible to variations caused by external factors. Due to these considerations, the use of probiotics for preventive or therapeutic agents is an established fact for some enteric diseases such as acute diarrhea and NEC, but it can be feasible for several diseases which are not apparently linked to the GIT microbiota composition, such as obesity and neurologic diseases. Unfortunately, most of the experimental evidences for these diseases regard in vitro studies, cell line experiments and, in some cases, animal experimental model researches. When these preliminary results are consolidated, clinical intervention trials using various strains possessing the GRAS and the QPS status can be planned to achieve definitive results on humans.
About Sanzyme Biologics:
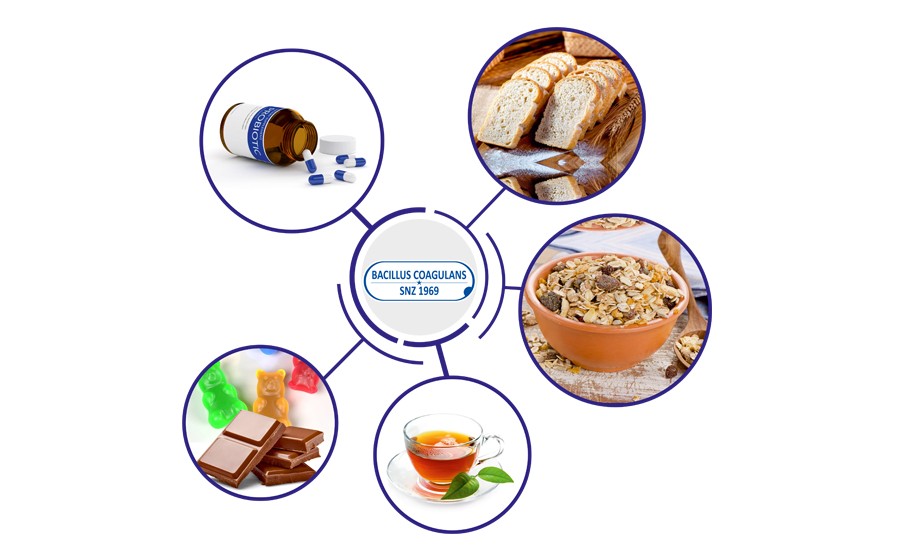
Sanzyme Biologics is a 50 year old biotechnology company based in Hyderabad, India. Sanzyme specialises in the production of probiotic bacteria. Sanzyme Biologics has obtained Generally Recognized as Safe (GRAS) approval relating to use of Bacillus coagulans SNZ 1969™ in infant formula. The US Food & Drug Administration (FDA) issued the company with a GRAS Notice known as a ‘no objection letter’. It states that it has no questions regarding the safety of Bacillus coagulans SNZ 1969™ spore preparation marketed by Sanzyme Biologics when used in infant formula.